1. Introduction:
Silane coupling agents are generally illustrated:
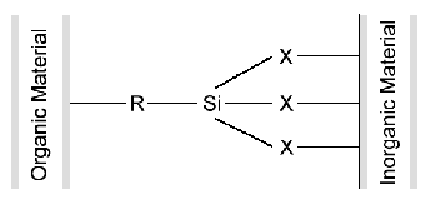
Silicone (Si) is
the center of the silane molecule which contains an organic functional
group (R) [ex: vinyl, amino, chloro, epoxy, mercapto, etc.], with
a second functional group (X) [ex: methoxy, ethoxy, etc.]. The functional
group (R) will attach to an organic resin while the functional group
(X) attaches to an inorganic material or substrate to achieve a
"coupling" effect.
Silane coupling agents are predominately
used as mediators, binding organic materials to inorganic materials.
As a result silanes will improve the electrical and mechanical strength
properties of materials in wet or dry conditions.
Silane coupling
agents are primarily used in reinforced plastics and electric cables
composed of crosslinked polyethylene. Other uses include resins,
concrete, sealant primers, paint, adhesives, printing inks and dyeing
auxiliaries.
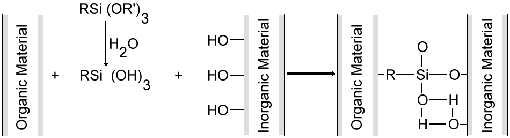
The inorganic group (X) of the
silane molecule will hydrolyze to produce silanol, which forms a
metal hydroxide or siloxane bond with the inorganic material. The
organic group (R) of the silane molecule will react with the organic
material to produce a covalent bond. As a result the organic material
and the inorganic material are tightly bound together after heating.
2a. Treating Process of Aqueous
Solutions of Silanes for Glass Fiber:
An aqueous solution of distilled
or deionized water (or a mixed solution of water and alcohol) and
0.1% to 0.5% silane coupling agent is prepared. The glass fiber
is dipped into the solution and dried at ambient temperature, followed
by heating at 110°C to 120°C for 5 to 10 minutes.
Fillers A filler which contains
an excess of hydroxyl groups on the surface is especially effective.
Very Effective: Silica, Alumina,
Glass, Quartz or Aluminum Silicate.
Moderately Effective: Talc, Hydrated Clay, Alumina, or Iron Powder.
Fairly Effective: Asbestos, Titanium Dioxide, and Zink Oxide.
1. Dry Process - The filler
is treated by spraying an aqueous solution of silane followed by
forced air or nitrogen to dry.
2. Wet Process - An aqueous solution of silane is added to a filler
dispersed in water. Following agitation the filler is allowed to
precipitate by separation and drying.
3. Spray Process - An aqueous solution of silane is sprayed on the
filler followed by heating. This process is simple and drying after
application is unnecessary.
2b. Silane Treating Process
for Metal, Ceramic or Glass Substrates:
The substrate is treated with
a mixed solution of 0.2% to 2.0% silane coupling agent and 98.0%
to 99.8% diluent (water/alcohol mixture or hydrocarbon). The dilution
could be applied by spray or immersion procedures, followed by drying
at 120oC to 180oC for 2 to 5 minutes. An aqueous solution of silane
is the most effective because the siliane hydrolyzes to form silanol.
3. Calculating the Quantity
of Silane Required:
The silicone molecule is preferably
attached to the surface of the inorganic material as a primer to
form a mono-layer. Applying a silane as a primer will produce optimum
coupling results between the substrate and the resin to be applied.
When used as a primer the required amount of silane can be calculated
by the following:

The actual values may deviate
from the calculated value depending on the surface condition of
the filler or the silane treating process. The following values
may be used as guidelines when the value is unknown. A dilution
of 1% silane to filler may be considered as standard. Generally
0.3% to 0.5% is recommended.
Surface area of
filler (m2/g)
E-glass |
0.1
- 0.12 |
Quartz |
1 -
2 |
Kolin |
7 |
Clay |
7 |
Talc |
7 |
Aluminum
polysilicate |
1 |
Calcium
carbonate |
5 |
Calcium
silicate |
2.6 |
4. Reactivity:
The alkoxy groups of the coupling
agents react with water to form silanol groups which immediately
form covalent bonds by dehydration and condensation:
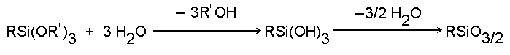
APPENDIX 1.
End-use Appilications
Benfits
Adhesives |
Moisture
initiated crosslinking of resins, improved wet adhesion, improved
chemical resistance, weatherability and filler/resin coupling. |
Coatings |
Moisture
initiated crosslinking of resins, improved wet adhesion, chemical
and corrosion resistance, weatherability, pigment dispersion
and scrub resistance. |
Crude Oil |
Extraction
Consolidation of down-hole fines |
Glass Fibers |
Coupling
of resins with fiber for improved resiliency of insulation
batts; better wet strength retention and electrical properties
of FRP composites, and improved fiber strand integrity, protection
and handling. |
Filler Treatment |
Improved
coupling of resins with fillers and bettter filler dispersion
in thermoset and thermoplastic resins.
Foundry Coupling of resins with sand for improved foundry
core strength. |
Polymer Modification |
Moisture-cure
crosslinking to give improved environmental and chemical resistance. |
Printing |
Inks Improved
adhesion, release and wetting. |
Rubber and
Elastomers |
Coupling
of resins with minerals for improved composite strength, toughness,
abrasion resistance, rolling resistance, wet electrical properties
and rheology control. |
Sealants |
Moisture
initiated crosslinking of resins, improved wet adhesion, chemical
resistance, filler dispersion, weatherability and rheology. |
Textiles |
Altered textile
hand and water repellency, and improved dye receptivity. |
Thermoplastics |
Moisture
curable XLPE for Wire & Cable and Pipe, Mineral and Pigment
treatment for dispersibility and coupling and reinforcement
coupling for high performance thermoplastics. |
NOTES: The choice
of a Power Silane is specific to resin type and application. The
Selection Guidelines is provided to help you select the appropriate
Power Silane for various polymer (resin) systems. It should be
considered merely a starting point. The selection of the preferred
silane for a specific end-use application may require specific
experimentation. |
 Power Chemical Corporation - SiSiB SILANES
Power Chemical Corporation - SiSiB SILANES Power Chemical Corporation - SiSiB SILANES
Power Chemical Corporation - SiSiB SILANES